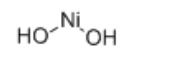
Identification
CAS Number
12054-48-7
Name
Nickel Hydroxide
Synonyms
12054-48-7 [RN]
235-008-5 [EINECS]
Dihydroxyde de nickel(2+) [French] [ACD/IUPAC Name]
MFCD00011140
Nickel(2+) dihydroxide [ACD/IUPAC Name]
Nickel(2+)dihydroxid [German] [ACD/IUPAC Name]
Nickel(II) hydroxide [Wiki]
QR7040000
[12054-48-7] [RN]
Ni(OH)2
Nickel dihydroxide
nickel hydroxide
Nickel hydroxide (amorphous)
Nickel hydroxide (Ni(OH)2)
Nickel hydroxide(ous)
NICKELDIOL
Nickelous hydroxide
SMILES
O[Ni]O
StdInChI
InChI=1S/Ni.2H2O/h;2*1H2/q+2;;/p-2
StdInChIKey
BFDHFSHZJLFAMC-UHFFFAOYSA-L
Molecular Formula
H2NiO2
Molecular Weight
92.708
EINECS
235-008-5
MDL Number
MFCD00011140
Properties
Appearance
Green powder
Safety Data
Symbol
Signal Word
Warning
Hazard statements
H302 + H332 – H315 – H317 – H334 – H341 – H350i – H360D – H372 – H410Precautionary Statements
P202 – P273 – P280 – P301 + P312 – P302 + P352 – P308 + P313WGK Germany
3
MSDS Download
Specifications and Other Information of Our
Identification Methods
HNMR, HPLC
Ni %:
≥55.5
Co %:
5.0±0.3
Zn %:
1.0±0.3
Ca %:
≤0.03
Mg %:
≤0.03
Fe %:
≤0.01
Mn %:
≤0.01
Cd %:
≤0.005
Cu %:
≤0.005
Pb %:
≤0.01
Cr %:
≤0.005
NO3- %:
≤0.02
SO42- %:
≤0.30
CI- %:
≤0.01
H2O %:
≤1.00
D50 μm:
12±2
APPARENT DENSITY g/cm3
≥1.65
TAP DENSITY g/cm3
≥2.10
SURFACE AREA m2/g
6-15
FULL WIDTH AT HALF MAXIMUM
≥0.85
Shelf Life
1 year
Storage
Store at room temperature, sealed and away from light.
Known Application
Nickel Hydroxide is widely used as a positive electrode material in nickel-metal hydride (NiMH) batteries and nickel-cadmium (NiCd) batteries. It exhibits high energy storage density and longer cycle life, making these batteries widely used in portable electronic devices, power tools, and hybrid electric vehicles.
Nickel hydroxide plays an important role as a catalyst in chemical reactions. It can be used as a catalyst for oxidation reactions, such as the oxidation of alcohols to aldehydes or ketones. Additionally, it can be utilized in other organic synthesis reactions, such as alkane dehydrogenation and carbonylation reactions.
General View of Documents
Links
This product is developed by our R&D company Warshel Chemical Ltd (https://www.warshel.com/).
Quick Inquiry
Fill out our inquiry form and one of our experts will be in touch with you shortly.



![Structure of 1-[2-fluoro-6-(trifluoromethyl)benzyl]-5-iodo-6-methylpyrimidine-2,4(1H,3H)-dione CAS 1150560-54-5](https://www.watson-int.com/wp-content/uploads/2020/08/Structure-of-1-2-fluoro-6-trifluoromethylbenzyl-5-iodo-6-methylpyrimidine-241H3H-dione-CAS-1150560-54-5-150x150.png)