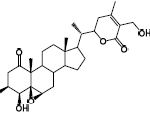
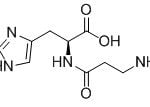
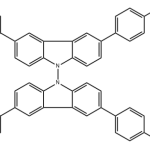
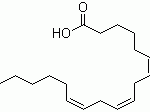
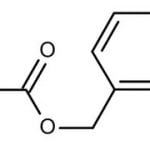
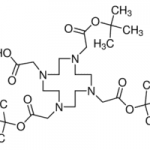
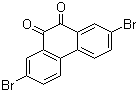
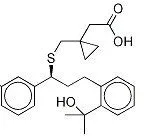
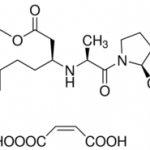
When you talk about chemical processes, most people are unaware of exactly how they tend to take place. One of the most important feature to keep in mind about chemical reactions is Catalysts. You must have heard of them throughout your life but what exactly are they? Well, in the simplest words, they are the agents responsible for doing nothing other than speeding up the reaction processes. Catalysts don’t really take part in the reactions, which means that they, themselves are not affected by the reactions taking place but they are a major part of them. If you’re not much aware of catalysts then you may not be aware of Catalysis either. This post will give you all the information you need about the process of Catalysis.
When you talk about chemical processes, most people are unaware of exactly how they tend to take place. One of the most important feature to keep in mind about chemical reactions is Catalysts. You must have heard of them throughout your life but what exactly are they? Well, in the simplest words, they are the agents responsible for doing nothing other than speeding up the reaction processes. Catalysts don’t really take part in the reactions, which means that they, themselves are not affected by the reactions taking place but they are a major part of them. If you’re not much aware of catalysts then you may not be aware of Catalysis either. This post will give you all the information you need about the process of Catalysis.Catalysis – What You Need to Know About It
 When you talk about chemical processes, most people are unaware of exactly how they tend to take place. One of the most important feature to keep in mind about chemical reactions is Catalysts. You must have heard of them throughout your life but what exactly are they? Well, in the simplest words, they are the agents responsible for doing nothing other than speeding up the reaction processes. Catalysts don’t really take part in the reactions, which means that they, themselves are not affected by the reactions taking place but they are a major part of them. If you’re not much aware of catalysts then you may not be aware of Catalysis either. This post will give you all the information you need about the process of Catalysis.
When you talk about chemical processes, most people are unaware of exactly how they tend to take place. One of the most important feature to keep in mind about chemical reactions is Catalysts. You must have heard of them throughout your life but what exactly are they? Well, in the simplest words, they are the agents responsible for doing nothing other than speeding up the reaction processes. Catalysts don’t really take part in the reactions, which means that they, themselves are not affected by the reactions taking place but they are a major part of them. If you’re not much aware of catalysts then you may not be aware of Catalysis either. This post will give you all the information you need about the process of Catalysis.
© 2025 WATSON INTERNATIONAL LIMITED. All Rights Reserved. This site and all content are protected by international Copyright laws. Third party logos and marks appearing on this website are trademarks and/or registered trademarks of their respective owners and licensors, and all rights therein and thereto are reserved to such entities.