 Identification
Properties
Safety Data
Specifications andamp ; Other Information
Links
Identification
Properties
Safety Data
Specifications andamp ; Other Information
Links
Identification
Name
LiODFB
CAS Number
409071-16-5
Synonyms
LiDFOB Lithium Oxalyldifluoro Borate Borate(1-), [ethanedioato(2-)-κO1,κO2]difluoro-, lithium (1:1) [
ACD/Index Name] Lithium [ethanedioato(2-)-κ2O1,
O2](difluoro)borate(1-) [
ACD/
IUPAC Name] 409071-16-5 [
RN] Lithium difluoro(oxalato)borate
LITHIUM DIFLUORO(
OXALATO)
BORATE(1-)
MFCD21608640 MFCD27952543 Molecular Structure
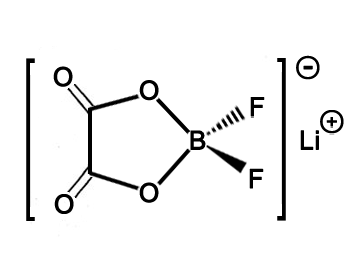
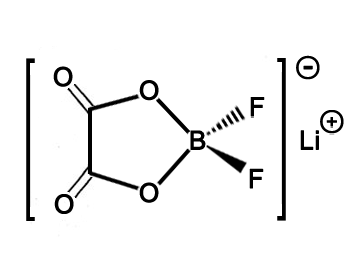
Structure of LiODFB CAS 409071-16-5
SMILES
[Li+].[B-]1(
OC(=O)C(=O)
O1)(F)F
StdInChI
InChI=
1S/
C2BF2O4.Li/c4-3(5)8-1(6)2(7)9-3;/q-1;+1
StdInChIKey
MEDDCIKGDMDORY-UHFFFAOYSA-N Molecular Formula
C2BF2LiO4
Molecular Weight
143.768
MDL Number
MFCD21608640 Properties
Appearance
White or light yellow powder
Melting Point
265-271 °C
Safety Data
Symbol
 GHS07
GHS07
Signal Word
Warning
Hazard Statements
H315-H319-H335
Precautionary Statements
P261-P305 + P351 + P338
RIDADR
NONH for all modes of transport
WGK Germany
3
MSDS
MSDS of LiODFB CAS 409071-16-5
Specifications and Other Information of Our LiODFB CAS 409071-16-5
Standard
Enterprise standard
Purity
99.8%min
Water
200ppm max
Insolubles
0.2% max
Na+K
20ppm max
Ca
5ppm max
Fe
5ppm max
Cl
5ppm max
SO4
5ppm max
Package
3kg/bottle
Storage
At room temperature or low temperature, dry and ventilated environment, sealed, away from heat
Features
- LiODFB has higher thermal stability and much less free acid content, and released CO2 possibly provides good safety of cells.
- The cells using LiODFB-based electrolyte have lower capacity fade than the cells using LiPF6-based electrolyte after 100 cycles at 55 ℃, and higher initial capacity retention at the elevated temperature.
- At 0.5C and 1C discharge rates, the rate capability of the cells with the LiODFB-based electrolyte is almost the same as that of the cells with the LiPF6-based electrolyte after 20 cycles,and the gap between the two cells is tiny.
- Interface properties of the cells show that LiODFB is reduced and forms a thickening and protective SEI film on the negative electrode. Although this can increase the impedance of SEI film, the cells still can provide a preferable rate performance. More work is needed to carry out in the lithium battery of commercialization in the future.
Application
For a secondary lithium ion battery or supercapacitor as a conductive salt or additives ; or for ionic liquids, pharmaceuticals and other organic synthesis Lithium oxalyldifluoroborate (LiODFB) is first reported as the salt for improved electrolyte of Li-ion battery. This salt was found to have the combined advantages of lithium bis(oxalato)borate (LiBOB) and LiBF4 due to its chemical structure comprising the half molecular moieties of LiBOB and LiBF4. Compared with LiBOB, the salt is more soluble in linear carbonates and the resulting solution is less viscous, which results in the battery better low temperature and high rate performance. Unlike LiBF4, the salt is highly capable of stabilizing solid electrolyte interface (
SEI) on the surface of graphite anode, which enables Li-ion cell to be operated stably at high temperature. For example, a graphite/LiNi1−x− yMxNyO2 (M and N are metal atoms) Li-ion cell suffered only about 10% capacity loss after 200 cycles at 60 °C. On the other hand, graphite can be cycled reversibly with LiODFB even in a solution containing high concentration (50 wt%) of propylene carbonate (
PC), which makes it possible to formulate the low freezing temperature electrolyte by using
PC as the co-solvent. Other merits of the LiODFB-based electrolytes include (1) the ability to support metallic lithium cycling reversibly on the surface of copper anode current collector, (2) the ability to passivate aluminum cathode current collector at high potentials, (3) the ability to participate in formation of the
SEI and support Li-ion battery operating stably at high temperatures, and (4) the ability to increase battery safety protection and overcharge tolerance.
Links
This product is developed by our
RD company Warshel Chemical Ltd(
http://www.warshel.com/), and here is the corresponding link
http://www.warshel.com/LiODFB-cas-409071-16-5/
Quick Inquiry
Fill out our inquiry form and one of our experts will be in touch with you shortly (Please change screen to horizontal for complete browsing if you are checking Watson on your mobile phone).
 Identification
Properties
Safety Data
Specifications andamp ; Other Information
Links
Identification
Properties
Safety Data
Specifications andamp ; Other Information
Links