- Identification
- Properties
- Safety Data
- Specifications & Other Information
- Links
- Quick Inquiry
Identification
CAS Number
164461-18-1
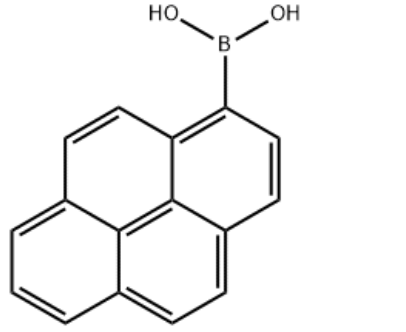
Name
1-Pyrenylboronic acid
Synonyms
164461-18-1 [RN]
1-Pyreneboronic acid
1-Pyrenylboronic acid [ACD/IUPAC Name]
1-Pyrenylborsäure [German] [ACD/IUPAC Name]
Acide 1-pyrénylboronique [French] [ACD/IUPAC Name]
Boronic acid, B-1-pyrenyl- [ACD/Index Name]
L666 B6 2AB PJ GBQQ [WLN]
MFCD04974062 [MDL number]
pyren-1-ylboronic acid
Pyrene-1-boronic acid
(pyren-1-yl)boronic acid
[164461-18-1] [RN]
1-Boronopyrene
1-Pyrene boronic acid
1-Pyreneboronic Acid (contains varying amounts of Anhydride)
1-Pyreneboronic Acid (en)
1-PyreneboronicAcid
1-Pyrenyl boronic acid
1-Pyrenylboronicacid
AGN-PC-0LMCQZ
BB-8011
Boronic acid, 1-pyrenyl-
GS-6482
POPULIN
PubChem16490
PYREN-1-YLBORONIC ACID|(PYREN-1-YL)BORONIC ACID
Pyrene-1-boronic acid|1-Boronopyrene
pyreneboronic acid
TL8001259
SMILES
B(c1ccc2ccc3cccc4c3c2c1cc4)(O)O
StdInChI
InChI=1S/C16H11BO2/c18-17(19)14-9-7-12-5-4-10-2-1-3-11-6-8-13(14)16(12)15(10)11/h1-9,18-19H
StdInChIKey
MWEKPLLMFXIZOC-UHFFFAOYSA-N
Molecular Formula
C16H11BO2
Molecular Weight
246.068
MDL Number
MFCD04974062
Properties
Appearance
Yellow powder
Structure
structure of 1-Pyrenylboronic acid CAS 164461-18-1
Safety Data
Signal Word
Warning
WGK Germany
3
MSDS Download
Specifications and Other Information of Our
Identification Methods
HNMR, HPLC
Purity
99.5% min
Lod
1%
Shelf Life
1 year
Storage
Cool, dry place with tighten contairier
Known Application
1-Pyrenylboronic acid can form coordination complexes with metal ions through the boronic acid group’s coordination ability. These complexes have important applications in catalysis, materials science, and bioanalysis.
1-Pyreneboronic acid can undergo carbon-carbon bond formation reactions with suitable reactants, such as organic halides or aromatic aldehydes. This reaction is often employed in organic synthesis for carbon-carbon bond connections and the construction of complex organic molecules. And due to the unique properties of the pyrene molecule, 1-Pyreneboronic acid is commonly used as a fluorescence probe or emitting group in photophysical and photochemical studies. Its fluorescence properties make it widely applicable in analytical chemistry, biology, and materials science.
General View of Documents
Links
This product is developed by our R&D company Warshel Chemical Ltd (https://www.warshel.com/).
Quick Inquiry
Fill out our inquiry form and one of our experts will be in touch with you shortly.