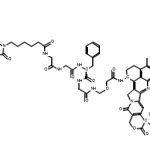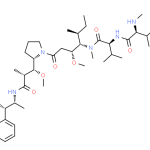
- Identification
- Properties
- Safety Data
- Specifications & Other Information
- Links
- Quick Inquiry
Identification
CAS Number
474645-27-7
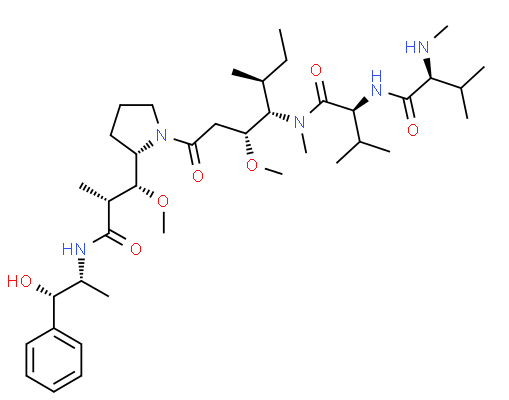
Name
MMAE, vedotin
Synonyms
474645-27-7 [RN]
L-Valinamide, N-methyl-L-valyl-N-[(1S,2R)-4-[(2S)-2-[(1R,2R)-3-[[(1R,2S)-2-hydroxy-1-methyl-2-phenylethyl]amino]-1-methoxy-2-methyl-3-oxopropyl]-1-pyrrolidinyl]-2-methoxy-1-[(1S)-1-methylpropyl]-4-oxo butyl]-N-methyl- [ACD/Index Name]
MMAE
Monomethyl auristatin E [Wiki]
Monomethylauristatin E
N-Methyl-L-valyl-N-[(3R,4S,5S)-1-{(2S)-2-[(1R,2R)-3-{[(1S,2R)-1-hydroxy-1-phenyl-2-propanyl]amino}-1-methoxy-2-methyl-3-oxopropyl]-1-pyrrolidinyl}-3-methoxy-5-methyl-1-oxo-4-heptanyl]-N-methyl-L-valin amid [German] [ACD/IUPAC Name]
N-Methyl-L-valyl-N-[(3R,4S,5S)-1-{(2S)-2-[(1R,2R)-3-{[(1S,2R)-1-hydroxy-1-phenyl-2-propanyl]amino}-1-methoxy-2-methyl-3-oxopropyl]-1-pyrrolidinyl}-3-methoxy-5-methyl-1-oxo-4-heptanyl]-N-methyl-L-valin amide [ACD/IUPAC Name]
N-Méthyl-L-valyl-N-[(3R,4S,5S)-1-{(2S)-2-[(1R,2R)-3-{[(1S,2R)-1-hydroxy-1-phényl-2-propanyl]amino}-1-méthoxy-2-méthyl-3-oxopropyl]-1-pyrrolidinyl}-3-méthoxy-5-méthyl-1-oxo-4-heptanyl]-N-méthyl-L-valin amide [French] [ACD/IUPAC Name]
UNII-V7I58RC5EJ
V7I58RC5EJ
(2S)-N-[(2S)-1-[[(3R,4S,5S)-1-[(2S)-2-[(1R,2R)-3-[[(1S,2R)-1-hydroxy-1-phenylpropan-2-yl]amino]-1-methoxy-2-methyl-3-oxopropyl]pyrrolidin-1-yl]-3-methoxy-5-methyl-1-oxoheptan-4-yl]-methylamino]-3-methyl-1-oxobutan-2-yl]-3-methyl-2-(methylamino)butanamide
(S)-N-((3R,4S,5S)-1-((S)-2-((1R,2R)-3-(((1S,2R)-1-hydroxy-1-phenylpropan-2-yl)amino)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidin-1-yl)-3-methoxy-5-methyl-1-oxoheptan-4-yl)-N,3-dimethyl-2-((S)-3-methyl-2
[474645-27-7] [RN]
4Q5
MFCD22124498 [MDL number]
MMAE (Monomethyl auristatin E)
Monomethyl auristatin E (MMAE)
N-methyl-L-valyl-N-[(1S,2R)-4-[(2S)-2-[(1R,2R)-3-[[(1R,2S)-2-hydroxy-1-methyl-2-phenylethyl]amino]-1-methoxy-2-methyl-3-oxopropyl]-1-pyrrolidinyl]-2-methoxy-1-[(1S)-1-methylpropyl]-4-oxobutyl]-N-methyl-L-valinamide
N-Methyl-L-Valyl-N-[(3r,4s,5s)-1-{(2s)-2-[(1r,2r)-3-{[(1s,2r)-1-Hydroxy-1-Phenylpropan-2-Yl]amino}-1-Methoxy-2-Methyl-3-Oxopropyl]pyrrolidin-1-Yl}-3-Methoxy-5-Methyl-1-Oxoheptan-4-Yl]-N-Methyl-L-Valinamide
UNII :V7I58RC5EJ
Untitled Document
Vedotin ; MMAE
SMILES
CC[C@H](C)[C@@H]([C@@H](CC(=O)N1CCC[C@H]1[C@@H]([C@@H](C)C(=O)N[C@H](C)[C@H](c2ccccc2)O)OC)OC)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](C(C)C)NC
StdInChI
InChI=1S/C39H67N5O7/c1-13-25(6)34(43(10)39(49)33(24(4)5)42-38(48)32(40-9)23(2)3)30(50-11)22-31(45)44-21-17-20-29(44)36(51-12)26(7)37(47)41-27(8)35(46)28-18-15-14-16-19-28/h14-16,18-19,23-27,29-30,32-36,40,46H,13,17,20-22H2,1-12H3,(H,41,47)(H,42,48)/t25-,26+,27+,29-,30+,32-,33-,34-,35+,36+/m0/s1
StdInChIKey
DASWEROEPLKSEI-UIJRFTGLSA-N
Molecular Formula
C39H67N5O7
Molecular Weight
717.979
MDL Number
MFCD22124498
Properties
Appearance
White powder
Melting Point
>90°C
Safety Data
RIDADR
NONH for all modes of transport
WGK Germany
3
Specifications and Other Information of Our MMAE, vedotin CAS 474645-27-7
Introduce
MMAE is widely used as a cytotoxic component to make antibody-drug conjugates (ADCs) to treat cancer. Due to its toxicity, it cannot be used as a drug by itself but rather linked to monoclonal antibodies (MABs). It is an effective anti-mitotic drug. It is a new type of drug synthesized from an extremely toxic polypeptide extracted from Aplysia in the Indian Ocean. It has a strong anti-tumor effect and is an anti-microbial drug like vinblastine. It is a tube drug, but its toxicity is about 200 times that of vinblastine. Because it is too toxic and cannot be used by intravenous drip, it can only exert its lethality in ADC drugs. It currently shows potent in vitro and in vivo activity against a variety of lymphomas, leukemias and solid tumors in preclinical studies.
Identification Methods
HNMR, HPLC
Purity
98% min
Shelf Life
2 years
Storage
Under room temperature away from light
Known Application
Monomethyl auristatin E (MMAE ; SGD-1010) is a synthetic derivative of Aplysia toxin 10, which acts as a potent mitotic inhibitor by inhibiting tubulin polymerization.
Links
This product is developed by our R&D company Caming Pharmaceutical Ltd (https://www.caming.com/).
Quick Inquiry
Fill out our inquiry form and one of our experts will be in touch with you shortly.