Identification
CAS Number
6556-12-3(70021-34-0)
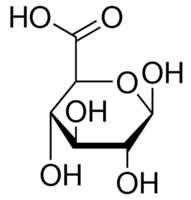
Name
D-Glucuronic acid
Synonyms
α-D-Glucopyranuronic acid [ACD/Index Name] [ACD/IUPAC Name]
6556-12-3 [RN]
70021-34-0 [RN]
Acide α-D-glucopyranuronique [French] [ACD/IUPAC Name]
D-Glucopyranuronic acid [ACD/Index Name] [ACD/IUPAC Name]
D-Glucuronic acid [ACD/Index Name] [ACD/IUPAC Name]
α-D-Glucopyranuronsäure [German] [ACD/IUPAC Name]
α-D-glucuronic acid
Glucosiduronate
Glucosiduronic acid
(2S,3S,4S,5R,6S)-3,4,5,6-tetrahydroxy-2-tetrahydropyrancarboxylic acid
(2S,3S,4S,5R,6S)-3,4,5,6-tetrahydroxyoxane-2-carboxylic acid
(2S,3S,4S,5R,6S)-3,4,5,6-tetrahydroxytetrahydro-2H-pyran-2-carboxylic acid
(2S,3S,4S,5R,6S)-3,4,5,6-tetrahydroxytetrahydro-2H-pyran-2-carboxylic acid ; α-D-glucopyranuronic acid
(2S,3S,4S,5R,6S)-3,4,5,6-tetrahydroxytetrahydropyran-2-carboxylic acid
03/12/6556
106499-29-0 [RN]
1285548 [Beilstein]
CHEBI:42717
D-(+)-glucuronate
D-(+)-glucuronic acid
D-Glucuronate [ACD/IUPAC Name]
GCU
glc
GlcAa
GlcAalpha
glucuronate [Wiki]
Glucuronic acid [Wiki]
α-D-Glucose
α-D-GLUCURONIC ACID
β-D-glucuronic acid
δ-glucuronate
葡糖醛酸 [Chinese]
SMILES
[C@@H]1([C@@H]([C@H](O[C@@H]([C@@H]1O)O)C(=O)O)O)O
StdInChI
InChI=1S/C6H10O7/c7-1-2(8)4(5(10)11)13-6(12)3(1)9/h1-4,6-9,12H,(H,10,11)/t1-,2-,3+,4-,6-/m0/s1
StdInChIKey
AEMOLEFTQBMNLQ–WAXACMCWSA-N
Molecular Formula
C6H10O7
Molecular Weight
194.139
EINECS
Beilstein Registry Number
1727083
MDL Number
Properties
Appearance
White crystalline powder
Safety Data
Personal Protective Equipment
Eyeshields, Gloves, type N95 (US), type P1 (EN143) respirator filter
RIDADR
NONH for all modes of transport
WGK Germany
3
RTECS
LZ8836600
Specifications and Other Information of Our D-Glucuronic acid CAS 6556-12-3(70021-34-0)
Identification Methods
HNMR, HPLC
Purity
98% min
Specific Optical Rotation
+11.7~+36.3°
Heavy Metals
≤1 ppm
Chloride
≤10 ppm
Loss on Drying
≤0.8%
Residue on Ignition
≤1 ppm
Sulfate
≤10 ppm
Arsenic
≤1 ppm
Nickel
≤0.5 ppm
Iron
≤1 ppm
Microorganism Total count
Not Detected
Yeast/Mould count
Not Detected
Escherichia coli
Not Detected
Storage
Under room temperature away from light
Known Application
D-Glucuronic acid is widely distributed in the plant and animal kingdoms. D-Glucuronic acid usually occurs form as a glycosidic combination with phenols, alcohols. Such glucuronides form in the liver to detoxify poisonous hydroxyl-containing substances. The glucuronides present in normal urine are those of phenol, cresol, and indoxyl. After the ingestion of poisons such as morphine, chloral hydrate, camphor, or turpentine, glucuronides formed with the poison or its hydroxylated derivatives appear in the urine.