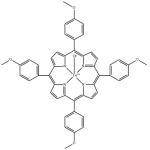
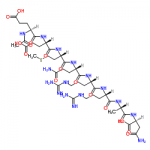
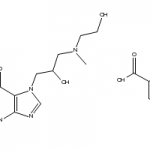
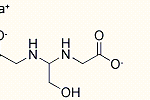
 Ionic liquids are salts in which ions are coordinated in a poor manner. This is why these solvents remain liquid below 100○C, as well as at room temperature. Ionic liquids are considered as green solvents and possess many unique qualities. One important aspect that should be kept in mind about ionic liquids is that they can be dramatically altered by impurities such as organic solvents, water and chloride ions. To learn about a chemical, it is important that its physical and chemical properties are known. Here are few significant physico-chemical properties of ionic liquids:
Ionic liquids are salts in which ions are coordinated in a poor manner. This is why these solvents remain liquid below 100○C, as well as at room temperature. Ionic liquids are considered as green solvents and possess many unique qualities. One important aspect that should be kept in mind about ionic liquids is that they can be dramatically altered by impurities such as organic solvents, water and chloride ions. To learn about a chemical, it is important that its physical and chemical properties are known. Here are few significant physico-chemical properties of ionic liquids:Physico-Chemical Properties of Ionic Liquids
 Ionic liquids are salts in which ions are coordinated in a poor manner. This is why these solvents remain liquid below 100○C, as well as at room temperature. Ionic liquids are considered as green solvents and possess many unique qualities. One important aspect that should be kept in mind about ionic liquids is that they can be dramatically altered by impurities such as organic solvents, water and chloride ions. To learn about a chemical, it is important that its physical and chemical properties are known. Here are few significant physico-chemical properties of ionic liquids:
Ionic liquids are salts in which ions are coordinated in a poor manner. This is why these solvents remain liquid below 100○C, as well as at room temperature. Ionic liquids are considered as green solvents and possess many unique qualities. One important aspect that should be kept in mind about ionic liquids is that they can be dramatically altered by impurities such as organic solvents, water and chloride ions. To learn about a chemical, it is important that its physical and chemical properties are known. Here are few significant physico-chemical properties of ionic liquids:
© 2025 WATSON INTERNATIONAL LIMITED. All Rights Reserved. This site and all content are protected by international Copyright laws. Third party logos and marks appearing on this website are trademarks and/or registered trademarks of their respective owners and licensors, and all rights therein and thereto are reserved to such entities.