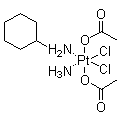 Identification
Properties
Safety Data
Specifications andamp ; Other Information
Links
Identification
Properties
Safety Data
Specifications andamp ; Other Information
Links
- About Watson
- Key Categories
- Formulations
- Dental Materials
- ChemWhat Products
- Synthetic Intermediates and Reagents
- Arylamines
- Naphthalenes / Acenaphthenes
- Anthracenes / Phenanthracenes
- Fluorenes / Fluoranthenes
- Pyrenes / Triphenylenes / Chrysenes
- Carbazole Derivatives
- Heterocyclics
- Iridium Complexes / Ligands
- Silanes Derivatives
- Thiophenes Derivatives
- Benzene Derivatives
- Boronic Acids / Boronic Esters
- Metal Catalysts / Reductants
- Organic Light-Emitting Diode (OLED) _ OLED Materials
- Organic Light-Emitting Diode (OLED) _ TADF Materials
- Solution-Processed OLED Materials _ Polymer
- Organic Photovoltaic (OPV)
- Photonic and Optical Device_Dyes
- Organic Thin-Film Transistor (OTFT) / Organic Field-Effect Transistor (OFET)
- Photonic and Optical Device
- Polyimide (PI)
- Organic Photodiodes (OPD) / Perovskite-based Photodiodes (PPD) __Organic Photodiodes
- Flexible Printed Electronics
- Synthetic Intermediates and Reagents
- Products & Industries
- Our Service & Capacity
- Download Brochures
- Contact Us
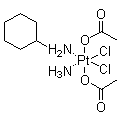 Identification
Properties
Safety Data
Specifications andamp ; Other Information
Links
Identification
Properties
Safety Data
Specifications andamp ; Other Information
Links
New Products
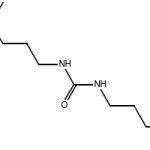 1,3-bis[3-(dimethylamino)propyl]urea CAS 52338-87-1Identification […]No Comments
1,3-bis[3-(dimethylamino)propyl]urea CAS 52338-87-1Identification […]No Comments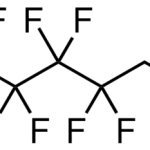 1H,1H,2H,2H-Perfluorooctanesulfonic acid CAS 27619-97-2Identification […]No Comments
1H,1H,2H,2H-Perfluorooctanesulfonic acid CAS 27619-97-2Identification […]No Comments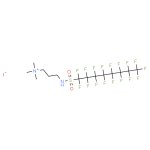 N,N-dimethyl,3-perfluorooctylsulfonylpropyl-aminium, iodide CAS 1652-63-7Identification […]No Comments
N,N-dimethyl,3-perfluorooctylsulfonylpropyl-aminium, iodide CAS 1652-63-7Identification […]No Comments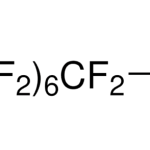 Perfluorooctanesulfonic acid potassium salt CAS 2795-39-3Identification […]No Comments
Perfluorooctanesulfonic acid potassium salt CAS 2795-39-3Identification […]No Comments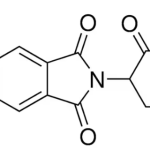 C5-Pomalidomide CAS 191732-76-0Identification […]No Comments
C5-Pomalidomide CAS 191732-76-0Identification […]No Comments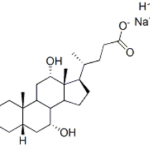 Sodium Cholate Hydrate CAS 73163-53-8Identification […]No Comments
Sodium Cholate Hydrate CAS 73163-53-8Identification […]No Comments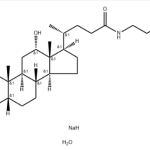 Sodium Tauro Deoxycholate Hydrate CAS 207737-97-1Identification […]No Comments
Sodium Tauro Deoxycholate Hydrate CAS 207737-97-1Identification […]No Comments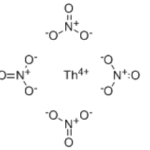 THORIUM NITRATE HYDRATE CAS 13823-29-5Identification […]No Comments
THORIUM NITRATE HYDRATE CAS 13823-29-5Identification […]No Comments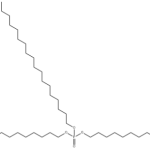 Trioctadecyl phosphate CAS 4889-45-6Identification […]No Comments
Trioctadecyl phosphate CAS 4889-45-6Identification […]No Comments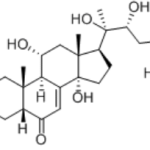 turkesterone CAS 41451-87-0Identification […]No Comments
turkesterone CAS 41451-87-0Identification […]No Comments
© 2024 WATSON INTERNATIONAL LIMITED. All Rights Reserved. This site and all content are protected by international Copyright laws. Third party logos and marks appearing on this website are trademarks and/or registered trademarks of their respective owners and licensors, and all rights therein and thereto are reserved to such entities.
