- Identification
- Properties
- Safety Data
- Specifications & Other Information
- Links
- Quick Inquiry
Identification
CAS Number
1190389-15-1
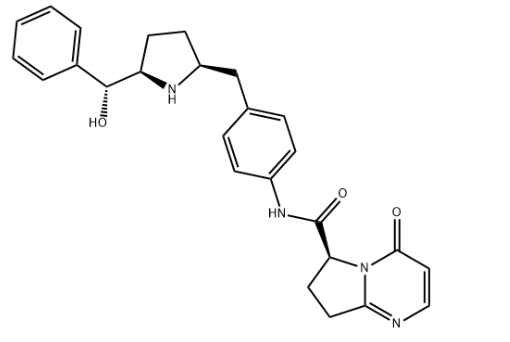
Name
Vibegron
Synonyms
(6S)-N-[4-({(2S,5R)-5-[(R)-Hydroxy(phenyl)methyl]-2-pyrrolidinyl}methyl)phenyl]-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-a]pyrimidin-6-carboxamid [German] [ACD/IUPAC Name]
(6S)-N-[4-({(2S,5R)-5-[(R)-Hydroxy(phenyl)methyl]-2-pyrrolidinyl}methyl)phenyl]-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-a]pyrimidine-6-carboxamide [ACD/IUPAC Name]
(6S)-N-[4-({(2S,5R)-5-[(R)-Hydroxy(phényl)méthyl]-2-pyrrolidinyl}méthyl)phényl]-4-oxo-4,6,7,8-tétrahydropyrrolo[1,2-a]pyrimidine-6-carboxamide [French] [ACD/IUPAC Name]
1190389-15-1 [RN]
Pyrrolo[1,2-a]pyrimidine-6-carboxamide, 4,6,7,8-tetrahydro-N-[4-[[(2S,5R)-5-[(R)-hydroxyphenylmethyl]-2-pyrrolidinyl]methyl]phenyl]-4-oxo-, (6S)- [ACD/Index Name]
Vibegron [USAN]
KRP-114V
MFCD28502057
SMILES
c1ccc(cc1)[C@H]([C@H]2CC[C@H](N2)Cc3ccc(cc3)NC(=O)[C@@H]4CCc5n4c(=O)ccn5)O
StdInChI
InChI=1S/C26H28N4O3/c31-24-14-15-27-23-13-12-22(30(23)24)26(33)29-19-8-6-17(7-9-19)16-20-10-11-21(28-20)25(32)18-4-2-1-3-5-18/h1-9,14-15,20-22,25,28,32H,10-13,16H2,(H,29,33)/t20-,21+,22-,25+/m0/s1
StdInChIKey
DJXRIQMCROIRCZ-XOEOCAAJSA-N
Molecular Formula
C26H28N4O3
Molecular Weight
444.53
Properties
Safety Data
WGK Germany
3
Specifications and Other Information of Our
Identification Methods
HNMR, HPLC
Known Application
Vibegron is a medication primarily used for the treatment of overactive bladder (OAB) and symptoms of urinary frequency and urgency. Overactive bladder is a common urological condition characterized by frequent and uncontrolled contractions of the bladder, leading to symptoms such as urinary frequency, urgency, and urinary incontinence.
Vibegron belongs to a class of drugs known as β3-adrenergic receptor agonists. It works by stimulating the β3-adrenergic receptors in the bladder wall, causing relaxation of the bladder smooth muscle, increasing bladder capacity, and reducing the frequency and intensity of bladder contractions. This action helps alleviate the symptoms of overactive bladder, improving the quality of life for patients.
It should be noted that Vibegron may cause some adverse reactions, including headache, nausea, diarrhea, high blood pressure and urinary tract infection. Before use, you should consult your doctor and learn more about the risks and contraindications of the drug.
Vibegron is typically prescribed by healthcare professionals based on the individual patient’s condition and symptoms. The dosage and administration should be adjusted according to the
General View of Documents
Links
This product is developed by our R&D company Caming Pharmaceutical Limited (https://www.caming.com/).
Quick Inquiry
Fill out our inquiry form and one of our experts will be in touch with you shortly.