Identification
CAS Number
9002-07-7
EC Number
3.4.21.4
Name
Recombinant trypsin
Synonyms
Trypsin-EDTA Solution 1X;
α-and β-trypsin;
Tryprar;
Trypsevas;
Trypsin Powder, Porcine 1:250;
TRYPSIN TYPE V-S:
ACETYLATED FROM*BOVINE PANCREAS;
TRYPSIN, PROTEOMICS SEQUENCING GRADE;
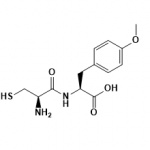
SMILES
CC1=CC(=CC2=C1C=CC(=O)O2)NC(=O)C(CCCN=C(N)N)NC(=O)C(CO)NC(=O)C(CC3=CC=CC=C3)NC(=O)OC(C)(C)C.CC(=O)O
InChI
InChI=1S/C33H43N7O8.C2H4O2/c1-19-15-21(17-26-22(19)12-13-27(42)47-26)37-28(43)23(11-8-14-36-31(34)35)38-30(45)25(18-41)39-29(44)24(16-20-9-6-5-7-10-20)40-32(46)48-33(2,3)4;1-2(3)4/h5-7,9-10,12-13,15,17,23-25,41H,8,11,14,16,18H2,1-4H3,(H,37,43)(H,38,45)(H,39,44)(H,40,46)(H4,34,35,36);1H3,(H,3,4)/t23-,24-,25-;/m0./s1
InChI Key
WGWZNYKOUXOZTC-NAGNLMCHSA-N
Molecular Formula
C35H47N7O10
Molecular Weight
725.78858
EINECS
232-650-8
MDL Number
MFCD00082094
Properties
Appearance
White or off white, or yellowish powder
Safety Data
Symbol
Signal Word
Danger
Hazard statements
H315 – H319 – H334 – H335Precautionary Statements
P302 + P352 – P305 + P351 + P338Target organs
Respiratory system
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
RIDADR
NONH for all modes of transport
WGK Germany
1
RTECS
YN5075000
Specifications and Other Information of Our Recombinant trypsin EC 3.4.21.4 CAS 9002-07-7
| Items | Recombinant Trypsin | Recombinant Trypsin (Liquid) | Recombinant Human Trypsin | Sequencing Grade Modified Trypsin |
| Appearance | White or off white, or yellowish powder | Clear, colorless or shallow yellow liquid | White or off white powder | White or off white, or yellowish powder |
| Protein Concentration | / | 70±10 mg/ml | / | / |
| Specific activity | ≥3800 USP units/mg pro. | ≥3800 (U/mg protein) | ≥2500 USP units/mg pro. | ≥4500 USP units/mg pro. |
| RP-HPLC | / | β-trypsin≥70%,α-trypsin ≤20% | / | ≥95% |
| E. Coli host Cell protein residue | / | ≤0.01% | / | / |
| Host Cell DNA residues | / | ≤ 10 ng/mg | / | / |
| Purity(SDS-PAGE) | Single major band | / | / | Single major band |
| Molecular Weight(SDS-PAGE) | 24.0±2.4 kDa | / | / | 24.0±2.4 kDa |
| Unit definition | One USP unit of trypsin activity will produce a Delta A253 of 0.003 per minute in a reaction volume of 3.2ml at pH7.6 and 25℃, with BAEE as a substrate (1cm light path). | One USP unit of trypsin activity will produce a Delta A253 of 0.003 per minute in a reaction volume of 3.2ml at pH7.6 and 25℃, with BAEE as a substrate (1cm light path). | / | / |
| Activity unit | 25℃, pH7.6, 3.2 ml reaction solution(1 cm light path), one trypsin unit (USP) was defined as an increase of 0.003 in the absorption value at 253 nm by enzymatic hydrolysis of BAEE per minute. | |||
| Recommended usage | The ratio to aimed protein is 1:50 to 1:1000 (w/w).The optimum pH is pH7.0-11.0. | Prepare 1-10mg/ml recombinant human trypsin .The ratio to aimed protein is 1:50 to 1:1000 (w/w).The optimum pH is pH7.0-11.0. | It is recommended to dissolve or dilute with 50 mM HAc. When used, dilute in 50 mM NH4HCO3 or ph7.0-8.0 buffer solution. 1 mM CaCl2 is recommended In the digestion buffer. The ratio to aimed protein is 1:50 to 1:1000 (w/w).The optimum pH is pH7.0-8.0. | |
| Stability of storage and transport | Recombinant trypsin lyophilized should be stored under 2-8℃ in sealed container. It is stable within 24 months.After dissolved with 50mM HAC, it should be stored under -20℃. It is no activity loss after 10 times repeated freezing and thawing. The product is stable by blue ice insulation transport. | The recombinant trypsin solution was stored under -20℃ and was stable for 12 months; Dry ice insulation transportation, won’t melt during transportation, stable activity. | Recombinant human trypsin lyophilized should be stored under 2-8℃ in sealed container. It is stable within 24 months.It is no activity loss after 10 times repeated freezing and thawing. The product is stable by blue ice insulation transport. | Sequencing grade trypsin lyophilized should be stored under 2-8℃ in sealed container.It is stable within 24 months.After dissolved with 1mM HCl or 50mM HAC, it should be stored under -20℃. It is no activity loss after 5 times repeated freezing and thawing. The product is stable by blue ice insulation transport. |
| Usage | The amino acid sequence of recombinant trypsin is identical to porcine pancreas-derived trypsin,with equivalent properties compared to native trypsin .Recombinant trypsin can replace native trypsin for using in a variety of biotechnological processes,such as in insulin and vaccine production. | For cell culture: Tissue block digestion, primary cell acquisition; Passage digestion of adherent cells; Cell culture by microcarrier method; Gently digesting stem cells; Immune cell therapy, etc. For recombinant protein: Recombinant insulin production; Protein sequencing, peptide mapping; Specific proteolytic processes such as proteomics research. | Recombinant human trypsin is an endopeptidase that can be used for lysine and arginine C-terminal cleavage of peptide bonds to cleave macromolecules into small peptides. Recombinant human trypsin is widely used in a variety of biotechnological processes, such as: cell culture cell separation of various tissues; degradation of denatured proteins; enzymatic hydrolysis, sequencing; stem cell therapy; tumor cell therapy. | The amino acid sequence of sequencing grade recombinant trypsin is identical to porcine pancreas-derived trypsin,with equivalent properties compared to native trypsin, and there are many advantages, such as no enzyme activity, high stability, non-self-cutting, high activity.When used, it is no non-specific fragments and self-cutting fragments.Sequencing grade recombinant trypsin can replace native trypsin for using in a variety of biotechnological processes,such as specific protein digestion, protein sequencing, peptide mapping, proteomics studies, Z-D peptides on the peptidase digestion. |
| Source | Recombinant trypsin is a genetically engineered protein expressed in E.coli | Recombinant Trypsin (Porcine), produced by genetic engineering, have the same amino acid sequence as the porcine pancreas trypsin. | Recombinant human trypsin is a genetically engineered protein expressed in E.coli | Sequencing grade modified trypsin is a genetically engineered methylation modified protein expressed in E.coli |
Advantage
- Animal origin free: recombinant trypsin is no exogenous virus contamination,and any animal origin material is not used in the production process.
- Stable quality: Mass production can ensure stable and continuous batch production.It is no difference between the batch and the product quality is stable.
- High purity: Higher specific activity.Host protein residues is less than the limits of biological products.
- Lyophilized powder: The product is lyophilized powder and is easy to store and transport.