- INTENDED USE
- SUMMARY
- PRINCIPLE
- REAGENTS
- PRECAUTIONS
- STORAGE AND STABILITY
- SPECIMEN COLLECTION AND PREPARATION
- MATERIALS
- DIRECTIONS FOR USE
- INTERPRETATION OF RESULTS
- QUALITY CONTROL
- PERFORMANCE CHARACTERISTICS
- SYMBOLS
- LINKS
- QUICK INQUIRY
INTENDED USE
COVID-19 IgG/IgM Rapid Test Device is a rapid chromatographic immunoassay for the qualitative detection of IgG & IgM antibody of COVID-19 IgM in human whole blood , serum, or plasma as an aid in the diagnosis of COVID-19 infections.
SUMMARY
Coronavirus (CoV) belongs to the genus Nestovirus, Coronaviridae, and is divided into three genera: α, β, and γ. The genus α and β are only pathogenic to mammals. The genus γ mainly causes bird infections. CoV is mainly transmitted through direct contact with secretions or through aerosols and droplets. There is also evidence that it can be transmitted through the fecal-oral route.
So far, there are 7 types of human coronavirus (HCoV) that cause human respiratory diseases: HCoV-229E, HCoV-OC43, SARS-CoV, HCoV-NL63, HCoV-HKU1, MERS-CoV and COVID-19, Is an important pathogen of human respiratory infections. Among them, COVID-19 was discovered due to Wuhan virus pneumonia cases in 2019. The clinical manifestations are systemic symptoms such as fever and fatigue, accompanied by dry cough and dyspnea, etc., which can rapidly develop into severe pneumonia, respiratory failure, and acute breathing. Distress syndrome, septic shock, multiple organ failure, severe acid-base metabolism disorders, etc. are even life-threatening.
PRINCIPLE
This kit uses immunochromatography. The test card contains: 1) colloidal gold-labeled recombinant new coronavirus antigen and quality control antibody gold markers; 2) two detection lines (G and M lines) and one quality Control line (C line) of nitrocellulose membrane. The M line is immobilized with a monoclonal anti-human IgM antibody for detecting a new coronavirus IgM antibody; the G line is immobilized with a reagent for detecting a new coronavirus IgG antibody; and the C line is immobilized with a quality control antibody.
When an appropriate amount of the test sample is added to the sample hole of the test card, the sample will move forward along the test card under the action of the capillary. If the sample contains an IgM antibody, the antibody will bind to the colloidal gold-labeled new coronavirus antigen. The immune complex will be captured by the anti-human IgM antibody immobilized on the membrane to form a purple-red M line, showing that the new coronavirus IgM antibody is positive.
If the sample contains an IgG antibody, the antibody will bind to the colloidal gold-labeled new coronavirus antigen, and the immune complex will be captured by the reagent immobilized on the membrane to form a purple-red G line, indicating that the new coronavirus IgG antibody is positive.
If the test lines G and M are not colored, a negative result is displayed. The test card also contains a quality control line C. The fuchsia quality control line C should appear regardless of whether a test line appears. The quality control line is a color band of the quality control antibody immune complex. If the quality control line C does not appear, the test result is invalid, and the sample needs to be tested again with another test card.
REAGENTS
The test contains COVID-19 virus envelope protein particles and anti-human IgG,anti-human IgM antibody conjugated gold particles coated on the membrane.
PRECAUTIONS
- For professional in vitro diagnostic use only. Do not use the kit beyond the expiration date.
- Do not eat, drink or smoke in the area where the specimens or kits are handled.
- Do not use the test if the pouch is damaged.
- Handle all specimens as if they contain infectious agents. Observe established precautions against microbiological hazards throughout testing and follow the standard procedures for proper disposal of specimens.
- Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are being tested.
- The used test should be discarded according to local regulations.
STORAGE AND STABILITY
- The original packaging should be stored at 4~ 30 ℃, to avoid light, keep dry.
- The test device is stable through the expiration date printed on the sealed pouch. The test device must remain in the sealed pouch until use.DO NOT FREEZE.
- Do not use beyond the expiration date, especially at temperatures above 30℃ or under high humidity conditions, should be used immediately once it is opened.
SPECIMEN COLLECTION AND PREPARATION
- The COVID-19 IgG/IgM Rapid Test Device is intended for use with human whole blood, serum or plasma specimens only.
- Only clear, non-hemolyzed specimens are recommended for use with this test. Serum or plasma should be separated as soon as possible to avoid hemolysis.
- Perform testing immediately after specimen collection. Do not leave specimens at room temperature for prolonged periods. Serum and plasma specimens may be stored at 2-8°C for up to 3 days. For long term storage, serum or plasma specimens should be kept below -20°C. Whole blood collected by venipuncture should be stored at 2-8°C if the test is to be run within 2 days after collection. Do not freeze whole blood specimens. Whole blood collected by fingerstick should be tested immediately.
- Containers containing anticoagulants such as EDTA, citrate, or heparin should be used for whole blood storage.
- Bring specimens to room temperature prior to testing. Frozen specimens must be completely thawed and mixed well prior to testing. Avoid repeated freezing and thawing of specimens.
- If specimens are to be shipped, pack them in compliance with all applicable regulations for transportation of etiological agents.
- Icteric, lipemic, hemolyzed, heat treated and contaminated sera may cause erroneous results.
MATERIALS
Materials Provided
| Test Devices | Buffer |
| Disposable plastic pipette | Package insert |
Materials Required But Not Provided
| Specimen collection containers | Centrifuge (for plasma only) |
| Micropipette | Timer |
| Lancets (for finger stick whole blood only) |
DIRECTIONS FOR USE
- Allow the test device, specimen, buffer, and/or controls to reach room temperature (15-30°C) prior to testing.
- Bring the pouch to room temperature before opening. Remove the test device from the sealed pouch and use it as soon as possible.
- Place the test device on a clean and level surface.
For Serum or Plasma Specimens:
Using the provided 10uL disposable pipette, draw the specimen up to the Fill Line, and transfer 10ul serum/plasma to the specimen well of the test device, then add 2 drops of buffer and start the timer.
For Whole Blood (Venipuncture/Fingerstick) Specimens:
Using the provided 10uL disposable pipette, and transfer 1 drop of whole blood (approximately20µL) to the specimen well of the test device, then add 2 drops of buffer and start the timer.
Note: Specimens can also be applied using a micropipette. - Wait for the colored line(s) to appear. Read results at 10 minutes. Do not interpret the result after 15 minutes.
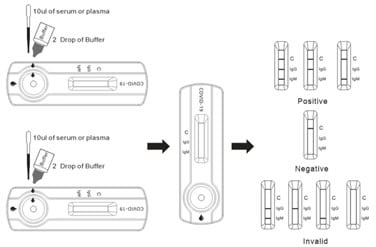


INTERPRETATION OF RESULTS
IgG POSITIVE: *The colored line in the control line region (C) appears and a colored line appears in test line region IgG. The result is positive for COVID-19-IgG antibodies.
IgM POSITIVE: *The colored line in the control line region (C) appears and a colored line appears in test line region IgM. The result is positive for COVID-19-IgM antibodies and is indicative of primary COVID-19 infection.
IgG AND IgM POSITIVE: *The colored line in the control line region (C) appears and two-colored lines should appear in test line regions IgG and IgM. The color intensities of the lines do not have to match. The result is positive for IgG & IgM antibodies.
*NOTE: The intensity of the color in the test line region(s) IgG and/or IgM will vary depending on the concentration of COVID-19 antibodies in the specimen. Therefore, any shade of color in the test line region(s) IgG and/or IgM should be considered positive.
NEGATIVE: The colored line in the control line region (C) appears. No line appears in test line regions IgG or IgM.
INVALID: There is no line appear in the c region.
Insufficient buffer volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the procedure with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
QUALITY CONTROL
Internal procedural controls are included in the test. A color line appearing in the control region (C) is an internal positive procedural control. It confirms sufficient specimen volume and correct procedural technique.
Control standards are not supplied with this kit; however, it is recommended that positive and negative controls be tested as a good laboratory practice to confirm the test procedure and to verify proper test performance.
PERFORMANCE CHARACTERISTICS
For Healthy Persons:
The 2019-nCOV IgG/IgM Rapid Test Device was compared with RT-PCR Reagent using clinical specimens from 100 healthy persons.
| Result | COVID-19 IgG Rapid | COVID-19 IgM Rapid | RT-PCR |
| Positive | 0 | 0 | 0 |
| Negative | 100 | 100 | 100 |
| Accuracy | 100% | 100% | 100% |
For Identified Persons:
The 2019-nCOV IgG/IgM Rapid Test Device was compared with RT-PCR Reagent using clinical specimens from 200 2019-nCOV identified patients.
| Result | COVID-19 IgG Rapid | COVID-19 IgM Rapid | RT-PCR |
| Positive | 192 | 181 | 200 |
| Negative | 8 | 19 | 0 |
| Accuracy | 96% | 90.5% | 100% |
For suspectable persons:
The 2019-nCOV IgG/IgM Rapid Test Device was compared with RT-PCR Reagent using clinical specimens from 200 suspectable 2019-nCOV patients, the results of RT-PCR are all negative.
| Result | COVID-19 IgG Rapid | COVID-19 IgM Rapid | RT-PCR |
| Positive | 149 | 140 | 0 |
| Negative | 51 | 60 | 200 |
| Accuracy | 74.5% | 70% | 0% |
| Notice: In our clinical report, all the data samples used in the file are real clinical ones. Our product has a high year-on-year detection rate in the market. In China, we have tested nearly 800 samples and only one false positive appeared. |
SYMBOLS
| Symbol | Meaning | Symbol | Meaning |
| In vitro diagnostic medical device | Storage temperature limit | ||
| Manufacturer | Authorized representative in the European Community | ||
| Date of Manufacture | Use by date | ||
| Do not reuse | Consult instruction for use | ||
| Batch code | Meet the requirements of EC Directive 98/79/EC |
LINKS
This product is developed by our R&D company Caming Pharmaceutical Ltd (httpS://www.caming.com/), and here is the corresponding link https://www.caming.com/covid-19-igg-igm-rapid-test-device/
QUICK INQUIRY
Fill out our inquiry form and one of our experts will be in touch with you shortly (Please change screen to horizontal for complete browsing if you are checking Watson on your mobile phone).