- New Coronavirus (COVID-19) Introduction
- Product Information of New Coronavirus (COVID-19) Antigen Rapid Test Kit (swab)
- Product Image
- Product Characteristics
- Intended Use
- Instruction For Use
- Specimen Collection
- Specimen preparation
- Test Process
- Schematic Diagram Of Test Process
- Interpretation of Assay Result
- Links
- Quick Inquiry
New Coronavirus (COVID-19) Introduction
The 2019 novel coronavirus, abbreviated as SARS-CoV-2, is a novel strain of coronavirus discovered in the human body. The symptoms of the virus are fever, fatigue, dry cough, and progressive dyspneal. In severe cases, the symptoms are acute respiratory distress syndrome, septic shock, metabolic acidosis and coagulation dysfunction that can’t be reversed. The virus has been confirmed the capacity of human-to-human transmission; the shortest incubation period of the virus is only 1 day, while the longest is 14 days.
Product Information of New Coronavirus (COVID-19) Antigen Rapid Test Kit (swab)
| Name | Specimen | Package | Storage Conditions |
| New Coronavirus (COVID-19) Antigen Rapid Test Kit (swab) | Nasal Swab and nasal aspirate samples | 25 Tests/box | 4-30°C storage in dark and dry |
Product Image









Product Characteristics
Test sample: Nasal Swab and nasal aspirate samples
Short detection time: Rapid detection of novel coronavirus within 15 minutes (SARS-CoV-2)
Instrumentation required: No need for instruments and equipment, suitable for rapid screening
Suitable for primary screening: Screening for suspected novel coronavirus patients
Intended Use
New (Novel) Coronavirus (COVID-19) Antigen Rapid Test (swab) is an in vitro diagnostic test for the qualitative detection of novel coronavirus antigens in Nasal Swab and nasal aspirate samples, using the rapid immunochromatographic method. The identification is based on the monoclonal antibodies specific for the New Coronavirus antigen. It will provide information for clinical doctors to prescribe correct medications.
Instruction For Use
Specimen Collection
- Nasal Aspiration
Collect nasal aspirate fluids using the specific aspirator as instructed. - Nasal Swabbing
Completely insert the sterilized swab supplied in this kit into the nasal basin, and swab several times to collect the epidermal cells of the mucus. It is recommended to collect sample from nasal basin for more accurate results.
Specimen preparation
Add 8 drops (about 0.24 mL) of the sample extraction buffer into the extraction tube supplied in this kit, and put it on the tube stand.
(1) Nasal Aspirate Fluids
Add 8 drops (about 0.24 mL) of the nasal aspirate fluids into the extraction tube which contains 0.3mL of the extraction buffer, and mix well to be used as test sample.
(2) Nasal Swabs
Insert the swab into the extraction tube which contains 8 drops (about 0.24 mL) of the extraction buffer. Rotate the swab inside the tube using a circular motion to roll the side of the extraction tube
so that liquid is expressed and reabsorbed from the swab. Remove the swab. The extracted solution will be used as test sample.
Test Process
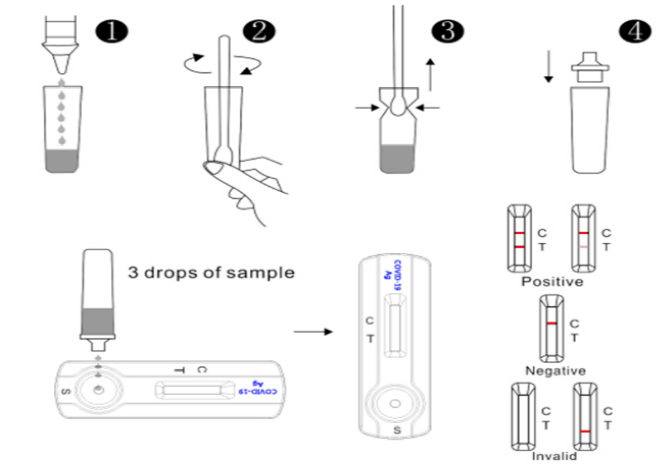
Test process
- Remove the test device from the sealed foil pouch and use it within 0.5 hour. Best results will be
obtained if the assay is performed immediately after opening the foil pouch - 1). Put the buffer vessel in an upright position.
2). Gently tap on the upper half of the vessel, ensure all buffer has flown to the bottom.
3). Twist the vessel neck until it breaks.
4). Place the vessel above the Extraction Tube, upside down. Squeeze the vessel hard until every drop of buffer has been transferred to the Extraction Tube. - Place the swab specimen in the Extraction Tube Rotate the swab for approximately 8 seconds while pressing the head against the inside of the tube to release the antigen in the swab.
- Remove the swab while squeezing the swab head against the inside of the Extraction Tube as you remove it to expel as much liquid as possible from the swab. Discard the swab in accordance with
your biohazard waste disposal protocol. - Fit the dropper tip on top of the extraction tube. Place the test device on a clean and level surface.
- Add three drops of the solution (approx. 80uL) to the sample well and then start the timer. Read the result at 15 minutes. Do not interpret the result after 20 minutes.
Schematic Diagram Of Test Process


Interpretation of Assay Result
- Positive: Two red lines appear. One red line appears in the control region (C), and one red line in the test region (T). The shade of color may vary, but it should be considered positive whenever there is even a faint line.
- Negative: Only one red line appears in the control region (C), and no line in the test region (T). The negative result indicates that there are no New Coronavirus particles in the sample or the number of
viral particles is below the detectable range. - Invalid: No red line appears in the control region (C). The test is invalid even if there is a line on test region (T). Insufficient sample volume or incorrect procedural techniques are the most likely reasons
for control line failure. Review the test procedure and repeat the test using a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
Links
This product is developed by our R&D company Watson Bio Ltd (https://www.watson-bio.com/), and here is the corresponding link https://www.watson-bio.com/new-coronavirus-covid-19-antigen-rapid-test-kit-swab/
Quick Inquiry
Fill out our inquiry form and one of our experts will be in touch with you shortly (Please change screen to horizontal for complete browsing if you are checking Watson on your mobile phone).