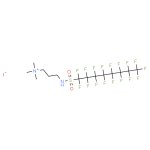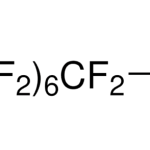
Double-layer capacitance allows storage of electrical energy using the electrical double layer effect. The phenomenon occurs between the interface of the electrode and electrolyte as seen, for instance, in supercapacitors. The electric charge stored through double layer capacitance is directly proportional to voltage. In supercapacitors, the capacitance mainly depends on electrode surface and DL distance. However, electrolyte mixture, electrode material and structure, and amount of pseudocapacitance also affect their capacitance to some extent. Research has time and again indicated effects of electrolyte composition on EDL and the potential of supercapacitors. Here are a few highlights to help determine the importance of composition.
Electrolyte Composition for EDL capacitance at Carbon Electrodes
Morita et al. (2006) investigated carbon electrodes of different surface morphologies with both organic and polymeric gel electrolytes1. The group found that both surface structure and electrolyte composition affected capacitance. For activated carbon fiber which had a pore structure, higher capacitance was achieved with polymeric gel compared to capacitance in the solution. In contrast, capacitance at smooth surfaced glassy carbon was higher in the solution compared to the polymeric gel. Also, the polymeric component offered different capacitance at the carbon electrode, varying with the surface morphology.EDL capacitance with TEABF4 and LiBF4
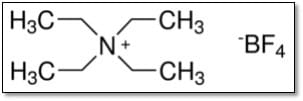 Akagi et al. demonstrated higher current response with Tetraethylammonium tetrafluoroborate (TEABF4) solution compared to Lithium tetrafluoroborate (LiBF4) solution for glassy carbon electrodes2. Moreover, the research also indicates higher specific capacitance with acetonitrile (AN) electrolyte solution compared to propylene carbonate (PC) solvent.
Akagi et al. demonstrated higher current response with Tetraethylammonium tetrafluoroborate (TEABF4) solution compared to Lithium tetrafluoroborate (LiBF4) solution for glassy carbon electrodes2. Moreover, the research also indicates higher specific capacitance with acetonitrile (AN) electrolyte solution compared to propylene carbonate (PC) solvent. Electrolyte Composition for activation of alkali-treated soft carbon
Ohta et al. demonstrated the activation of alkali-treated soft carbon (ASC) with varying compositions of organic electrolyte solutions3. Anodic polarization of TEABF4 solution resulted in higher capacitive currents in positive potential region. Cathodic polarization of TEABF4 solution resulted in increased currents in cation adsorption/desorption. Lastly, sizes of solvent molecules and ions affected activation especially at the cathode. Larger ions and molecules were more effective while smaller were preferable for higher capacitance.EDL electrolytes at Watson International
Renewable energy is the future. Supercapacitors are primarily adopted by automotive industries for hybrid electric vehicles, increasing interest in electrolytes that promise higher capacitance. We offer a number of supercapacitor electrolytes, including Tetraethylammonium tetrafluoroborate, TEABF4. Here are selected electrolytes that you can order right away !- Tetraethyl ammonium tetrafluoroborate : http://watson-int.com/teabf4-acn-cas-429-06-1/
- Triethylmethylammonium tetrafluoroborate : http://watson-int.com/temabf4acn-cas-69444-47-9/
- Succinimidyl-[4-(psoralen-8-yloxy)]-butyrate : http://watson-int.com/sbpbf4-acn-cas-129211-47-8/
References
- Morita, Masayuki, et al. “Effects of the electrolyte composition on the electric double-layer capacitance at carbon electrodes.” Electrochemical and solid-state letters8 (2006): A386-A389.
- Akagi, Shogo, et al. “Effects of Electrolyte Composition on the Double Layer Capacitance at a Smooth Surface Carbon Electrode.” Meeting Abstracts. No. 5. The Electrochemical Society, 2008.
- Ohta, Tomoki, et al. “Effects of electrolyte composition on the electrochemical activation of alkali-treated soft carbon as an electric double-layer capacitor electrode.” Journal of Power Sources198 (2012): 408-415.