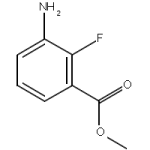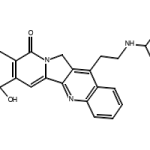
Two things that severely impact the reaction rate are catalysts and enzymes. By and large, almost all enzymes known to mankind today are catalysts, but all catalysts aren’t necessarily enzymes. To understand the difference on a basic level, enzymes are untreated by nature and tend to be bio-catalysts. On the other hand, catalysts that are not enzymatic are primarily treated or inorganic by nature. Moreover, the reactions catalyzed by enzymes and catalysts do not absorb them during the process. Keep reading below to uncover how different catalysts and enzymes are from one another and the role they play in chemical reactions :
The Structure
Catalysts are substances that can lead to major modifications to the rate at which chemical reactions take place. From an element as pure as nickel or platinum, to an unadulterated compound such as Silica – it can be just about anything. The most frequently utilized catalysts are proton acids that are used in a hydrolysis process. On the other hand, enzymes are spherical proteins and comprise of amino acids ranging from 62 to 2,500 amino acids. Enzymes are also found in RNA-based enzymes referred to as ribozymes. Typically, the enzymes are specific to substances on which they act and are generally bigger relative to their individual substrates.
The Function
Catalysts are primarily known to alter (increase or decrease) the tempo of chemical reactions. However, they themselves remain unaltered throughout the chemical process. Possessing a low molecular weight, catalysts are generally of two types : positive catalysts and negative catalysts. Enzymes, on the contrary, are basically proteins that only enhance or improve the rate of chemical reactions. They do so by transitioning the substrate into a product. Possessing a high molecular weight, enzymes are typically of two kinds : activation enzymes and inhibitory enzymes.
The Mechanism of their Reactions
As stated earlier,
catalysts increase or decrease the velocity of a reaction. They reaction takes place then the reactants in generate intermediates that launch the product and reproduce the catalyst. Let’s consider an example below :
W= Catalyst, X and Y = Reactants, and P = Product. A catalytic chemical reaction would go this way :
X+W = XW,
Y+W = YW,
XYW = PW,
PW = P+W. On the other hand,
enzymatic reactions take place in more than one way. Some of them include :
- Reducing the activation energy and increasing the steady evolution state typically obtained by deforming the substrate’s shape,
- Reducing the changeover condition energy with no distortion to the substrate,
- Provisional development of enzyme substrate compound, and thus offering another conduit for the reaction to ensue,
- Reduction in the reaction’s entropy,
- Mounting temperature.
Find an array of
enzymes and
catalysts at Watson International Ltd. We also stock a range of compounds and chemicals such as
Fullerene C60 99685-96-8,
Methylpentanoic acid and more. Call us at 001 909 945 0760-31 to learn more about our available products.

 Two things that severely impact the reaction rate are catalysts and enzymes. By and large, almost all enzymes known to mankind today are catalysts, but all catalysts aren’t necessarily enzymes. To understand the difference on a basic level, enzymes are untreated by nature and tend to be bio-catalysts. On the other hand, catalysts that are not enzymatic are primarily treated or inorganic by nature. Moreover, the reactions catalyzed by enzymes and catalysts do not absorb them during the process. Keep reading below to uncover how different catalysts and enzymes are from one another and the role they play in chemical reactions :
Two things that severely impact the reaction rate are catalysts and enzymes. By and large, almost all enzymes known to mankind today are catalysts, but all catalysts aren’t necessarily enzymes. To understand the difference on a basic level, enzymes are untreated by nature and tend to be bio-catalysts. On the other hand, catalysts that are not enzymatic are primarily treated or inorganic by nature. Moreover, the reactions catalyzed by enzymes and catalysts do not absorb them during the process. Keep reading below to uncover how different catalysts and enzymes are from one another and the role they play in chemical reactions :